-
Baoan District, Shenzhen, Guangdong, China

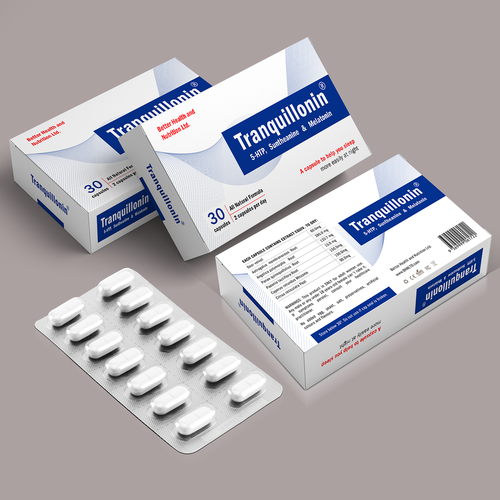

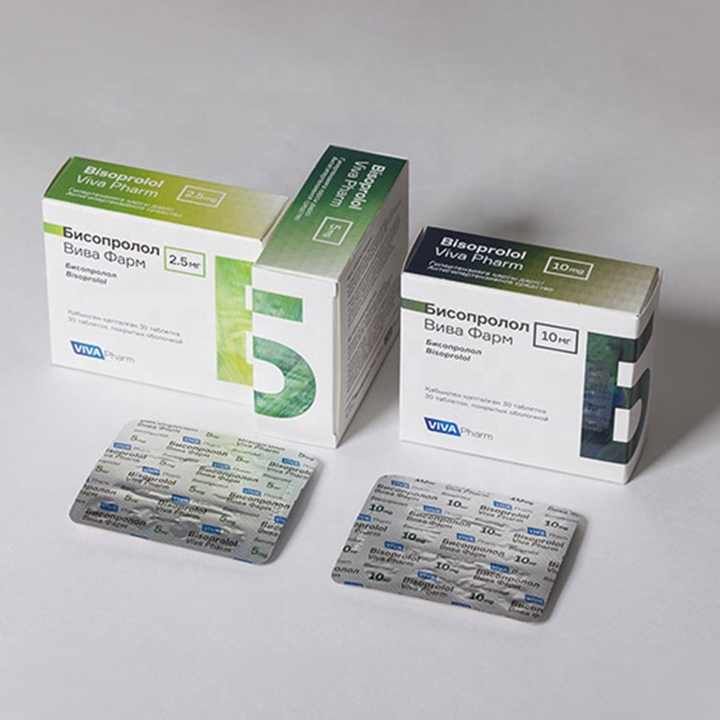
Pharmaceutical Packaging Paper Boxes
- Material: 300g coated paper + F-flute
- Size: Custom
- MOQ: 1000pcs
Free Sample / Factory Price / Bulk Customization / Logo & Packaging / Wholesale & Export /
Pharmaceutical packaging paper boxes are specifically designed and manufactured to safely contain and protect pharmaceutical products. These boxes are critical for maintaining the quality, integrity, and safety of medications and medical supplies. Here are some key aspects and considerations related to pharmaceutical packaging paper boxes:
1. Material Selection:
- Pharmaceutical paper boxes are typically made from high-quality, pharmaceutical-grade paperboard or cardboard. The choice of material should ensure product protection, stability, and compliance with industry regulations.
2. Durability and Strength:
- These boxes must be durable enough to protect the contents during handling, storage, and transportation. They should resist moisture, light, and other environmental factors that could affect pharmaceuticals.
3. Customization:
- Pharmaceutical packaging boxes can be customized to meet the specific needs of different medications and products. Customization includes sizing, design, printing, and branding.
4. Compliance with Regulations:
- Pharmaceutical packaging must adhere to stringent regulatory requirements, such as those set by the FDA (U.S. Food and Drug Administration) or equivalent agencies in other countries. This ensures that the packaging is safe for storing medications.
5. Child-Resistant Packaging:
- For certain medications, particularly those that pose a risk to children, pharmaceutical paper boxes may need to incorporate child-resistant features or closures to prevent accidental ingestion.
6. Tamper-Evident Features:
- To protect against tampering or contamination, pharmaceutical packaging often includes tamper-evident features like seals or breakable tabs. These features indicate if the package has been opened or compromised.
7. Information and Labeling:
- Pharmaceutical boxes should include all necessary labeling information, such as product name, dosage instructions, active ingredients, expiration date, lot number, and manufacturer details.
8. Barcoding and Serialization:
- Many pharmaceutical products require unique barcodes and serial numbers for tracking and traceability purposes. The packaging should accommodate these identifiers.
9. Adherence and Compliance Aids:
- Some pharmaceutical packaging designs include features to help patients adhere to medication regimens, such as blister packs or pill organizers.
10. Temperature Sensitivity: – For temperature-sensitive medications, consider packaging solutions that offer insulation or thermal protection to maintain product efficacy.
11. Eco-Friendly Options: – As sustainability becomes more important in the pharmaceutical industry, consider using eco-friendly materials and printing processes for packaging.
12. Quality Control: – Implement rigorous quality control processes to ensure that every pharmaceutical paper box meets the highest quality and safety standards.
13. Batch and Lot Information: – If pharmaceutical products are packaged in batches or lots, the boxes should clearly display this information for tracking and recalls if necessary.
14. Anti-Counterfeiting Measures: – In regions where counterfeit pharmaceuticals are a concern, packaging may include anti-counterfeiting features, such as holograms or specialized inks.
15. Patient Safety: – Prioritize patient safety by designing packaging that minimizes the risk of dosage errors or confusion. Clear labeling and user-friendly designs can contribute to this.
Pharmaceutical packaging paper boxes play a critical role in ensuring the safety, efficacy, and compliance of medications. Manufacturers must carefully consider regulatory requirements, product-specific needs, and user-friendliness when designing and producing these boxes to meet the highest industry standards.
| Type | Round, Other |
|---|---|
| Material | Carboard |
One Stop Printing & Packaging Service
RENHE is a leading manufacturer in the packaging printing industry. With quality services and products, we have earned the appreciation of our customers worldwide over the past 30 years.
We provide one-stop package & printing customization solutions, our professional R&D, QC team and abundant production lines to ensure production can be smooth and delivered on time. Welcome customers worldwide to contact us or request a free quote.








